 Transcranial magnetic stimulation (TMS) applies gentle magnetic pulses (similar to the magnetic field used in an MRI machine) to targeted areas of the brain. Stimulating the brain in this way enhances “neuroplasticity,” or the brain’s ability to change itself, and helps restore normal function. TMS works differently than medications, and is more effective than medication for many patients. TMS does not affect other areas of the body, so for most people, it has fewer side effects than medications.
Transcranial magnetic stimulation (TMS) applies gentle magnetic pulses (similar to the magnetic field used in an MRI machine) to targeted areas of the brain. Stimulating the brain in this way enhances “neuroplasticity,” or the brain’s ability to change itself, and helps restore normal function. TMS works differently than medications, and is more effective than medication for many patients. TMS does not affect other areas of the body, so for most people, it has fewer side effects than medications.
TMS was FDA approved for the treatment of Major Depressive Disorder in 2008 and is also FDA approved for Obsessive-Compulsive Disorder (OCD). Studies suggest that TMS is also helpful for anxiety disorders, Post-Traumatic Stress Disorder (PTSD), chronic pain, tinnitus, and other conditions.
TMS for Major Depressive Disorder is covered by most major insurance plans and is endorsed by the American Psychiatric Association.
 TMS is a safe and highly effective treatment. Overall, about two in three patients with depression will benefit noticeably from TMS treatment. TMS reduces symptoms of depression by an average of 40% or more. Many people begin to notice benefit after just 1 or 2 weeks of treatment.
TMS is a safe and highly effective treatment. Overall, about two in three patients with depression will benefit noticeably from TMS treatment. TMS reduces symptoms of depression by an average of 40% or more. Many people begin to notice benefit after just 1 or 2 weeks of treatment.
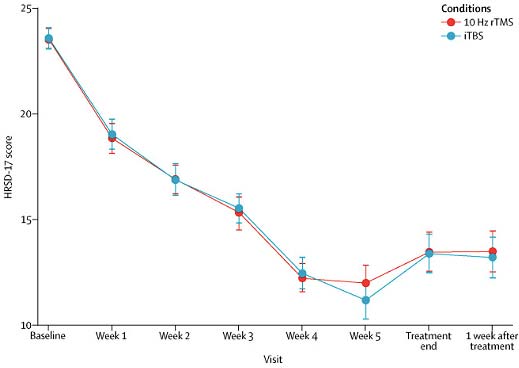
This figure (DM Blumberger et al, 2018) demonstrates the average decrease in depression rating scores over 6 weeks of treatment for patients receiving standard treatment (“10 Hz,” 37 minutes per session) and patients receiving a newer, shorter treatment (“theta burst,” as little as 3 minutes per session)
TMS works well for patients who have not responded to medication; in fact, patients who have not responded well to 1 or more medications have a higher chance of responding to TMS than to a second or third medication.
TMS is FDA approved and has an excellent track record of safety over the past decade. Some patients experience mild discomfort during the treatment, or mild headaches or fatigue afterwards. For most people, discomfort, headaches, and fatigue improve or resolve after the first few treatments. The only serious potential side effect of TMS is an extremely low risk of seizure (less than 0.01%).
TMS might be right for you if you have any of the following conditions:
We sometimes treat other conditions on a case-by-case basis after performing a comprehensive evaluation and risk-benefit analysis. Please reach out directly to learn more.
You may not be a good candidate for TMS if you have seizures, epilepsy, or any type of metal in your head or neck (such as aneurysm clips, stents, deep brain stimulators, cochlear implants, shrapnel, or others). If you have any of these conditions, we can perform a comprehensive evaluation to determine whether you may still be eligible for treatment.
You have many options for TMS treatment in Southern California. The table below may help you compare our approach to that of other providers you are considering.
| UCLA TMS | Other TMS Providers | |
|---|---|---|
| Comprehensive assessment for TMS, at-home neuromodulation, ketamine, and other treatment options | checkmark | |
| Development of a personalized treatment plan | checkmark | |
| Measurement-based care with weekly treatment planning meetings | checkmark | |
| Meeting with a psychiatrist at every treatment session in order to discuss your treatment progress | checkmark | |
| Regular communication from our treatment team to your primary mental health care provider | checkmark | |
| Sophisticated treatment protocols informed by the latest research | checkmark | |
| Customized adjustments to your treatment plan as often as needed to maximize your benefit | checkmark | |
| Combined treatment for OCD, pain, and other conditions that occur with depression, at no additional cost to you | checkmark | |
| Evidence-based and affordable treatment of chronic pain, tinnitus, and other conditions usually not covered by insurance | checkmark | |
| Affordable relapse prevention treatment protocols for depression | checkmark |